Personal genomics: ACTN3: Difference between revisions
Tomemerald (talk | contribs) |
Tomemerald (talk | contribs) |
||
| Line 113: | Line 113: | ||
=== R577x and co-evolution of actinin spectrin repeats === | === R577x and co-evolution of actinin spectrin repeats === | ||
( | |||
The four alpha actinin paralogs in human all have the same domain structure, namely 2 calponin-homology domains (that bind actin) followed by 4 spectrin rod domains followed by 2 EF hands that ancestrally bound calcium. These domains comprise almost the entirety of the 901 amino acids of ACTN3. All three domain types are exceedingly common in hundreds of human proteins in various permutations, associations, and repeat numbers. | |||
Actinins are a classic chimeric domain protein. The roles of each domain types are fairly well-known and excellent 3D domain structures are available; as an approximation, these domain folds independently.Actinins form anti-parallel dimers via their spectrin domains, meaning the overall shape is that of a dumbbell. Dimerization (ie two binding sites) is essential to the basic actin cross-linking functionality of actinins. ACTN3 and ACTN2 can form heterodimers. Related proteins such as dystrophin with similiar chains of spectrin domains could conceivably interact with actinins in a similar manner. | |||
In many domain-shuffled proteins (eg titin), the number of internal repeats is both evolutionarily unstable (due to the intrinsic propensity for mis-aligned recombination or replication slippage) and subject to domain-skipping alternative splicing, seemingly without drastic end-functional consequences. Such chimeric proteins are notoriously difficult to align homologically (ie by evolutionary descent) because quite different histories of contraction and expansion can result in same domain copy position. | |||
Here it must be noted that splice boundaries in domain-shuffled proteins (21 coding exons in all actinins) do not necessarily allow for clean excision of domains, apparently depending on the relative timing of protein origin relative to the main era of intron gain. It appears that the ancestral actinin gene (with its internal domain repeat expansion complete) arose very early, prior to exonization, whereas its segmental gene duplications to vertebrate paralogs took place much later, subsequent to exonization, yet not so recently that any flanking gene synteny nor any sequence alignability across spectrin domains could be retained. | |||
When actinins from early eukaryotes are [http://mbe.oxfordjournals.org/cgi/content/full/24/10/2254 considered], it emerges that SP1 is likely the ancestral spectrin domain (one such domain still suffices for dimerization). An internal duplication prior to fungal divergence gave rise to SP4 and a protein with two spectrin repeats. SP2 and SP3 apparently arose from a second intragenic divergence, giving rise to the four spectin repeats seen in all extant vertebrate actinins. | |||
The number of repeats determine the length of spacer, total binding strength, and access to other binding proteins such as [http://www.ncbi.nlm.nih.gov/pubmed/16450054 fessilin]. Note the spectrin gene SPTA1 contains 22 spectrin domains, so the 4 of actinins is not exceptional. | |||
The domain composition of ACTN3 is shown relative to its exons below, followed by a comparison of ACTN gene family chromosomal contexts. | |||
>ACTN3_homSap Homo sapiens (human) 21 exons 901aa NM_001104 <font color="#CC00FF">Q523R</font> <font color="#FF0000">R577x</font> <font color="#CC00FF">R628C</font> <font color="#CC00FF">R776Q</font> <font color="#996633">CH1</font> <font color="#66CC66">Ch2</font> <font color="#009900">SP1</font> <font color="#CC9966">SP2</font> <font color="#0066CC">SP3</font> <font color="#990099">SP4</font> <font color="#00CC66">EF1</font> <font color="#999900"EF2</font> | |||
0 MMMVMQPEGLGAGEGRFAGGGGGGEYMEQEEDWDRDLLLDPAW<font color="#996633">EKQQRK 0 | |||
0 TFTAWCNSHLRKAGTQIENIEEDFRNGLKLMLLLEVIS 1 | |||
2 GERLPRPDKGKMRFHKIANVNKALDFIASKGVKLVSIGAE 1 | |||
2 EIVDGNLKMTLGMIWTIILRFA</font>IQDISVE 1 | |||
2 E<font color="#66CC66">TSAKEGLLLWCQRKTAPYRNVNVQNFHTS 2 | |||
1 WKDGLALCALIHRHRPDLIDYAKLR 0 | |||
0 KDDPIGNLNTAFEVAEKYLDIPKMLDAE 1 | |||
2 DIVNTPKPDEKAIMTYVSCFYH</font>AFAGAEQ 0 | |||
0 AETAANRICKVLAVNQENE<font color="#009900">KLMEEYEKLASE 0 | |||
0 LLEWIRRTVPWLENRVGEPSMSAMQRKLEDFRDYRRLHKPPRIQEKCQLEINFNTLQTKLRLSHRPAFMPSEGKLVS 0 | |||
0 DIANAWRGLEQVEKGYEDWLLS</font>EIRRLQRLQ<font color="#CC9966">HLAEKFRQKASLHEAWTR 1 | |||
2 GKEEMLSQRDYDSALLQEVRALLRRHEAFESDLAAHQDRVEHIAALAQELN 2 | |||
1 ELDYHEAASVNSRCQAICDQWDNLGTLTQKRRDALE 0 | |||
0 R</font>MEKLLETID<font color="#CC00FF">Q</font><font color="#0066CC">LQLEFARRAAPFNNWLDGAVEDLQDVWLVHSVEETQ 0 | |||
0 SLLTAHDQFKATLPEAD</font><font color="#FF0000">R</font><font color="#0066CC">ERGAIMGIQGEIQKICQTYGLRPCSTNPYITLSPQDINTKWDM 0 | |||
0 VRKLVPS</font><font color="#CC00FF">R</font><font color="#0066CC">DQTLQ</font>EELARQQVNE<font color="#990099">RLRRQFAAQANAIGPWIQAKVE 0 | |||
0 EVGRLAAGLAGSLEEQMAGLRQQEQNIINYKTNIDRLEGDHQLLQESLVFDNKHTVYSME 0 | |||
0 HIRVGWEQLLTSIARTINEVEN</font>QVLTRDAKGLSQ<font color="#00CC66">EQLNEFRASFNHFDR 0 | |||
0 K</font><font color="#CC00FF">R</font><font color="#00CC66">NGMMEPDDFRACLISMGYD</font><font color="#999900">L 0 | |||
0 GEVEFARIMTMVDPNAAGVVTFQAFIDFMTRETAE</font>TDTTEQVVASFKILAGDK 0 | |||
0 NYITPEELRRELPAKQAEYCIRRMVPYKGSGAPAGALDYVAFSSALYGESDL* 0 | |||
Spectrin repeats (independently folding rodlike structural spacers without catalytic, post-translational modifications, regulatory substrate, or non-homlogous protein binding sites) might seem especially subject to these problems. However that cannot be not the case in actinins because the proteins invariably occur as dimers joined along the spectrin interface. This forces a two-fold symmetry axis per standard theories of protein oligomericity (fully supported by crytallographic studies), meaning anti-parallel association of spectrin domains. | |||
In other words, SP1 of one actinin monomer binds to SP4 of the other and SP2 to SP3 (ie 1234 to 4321). That has an additional twist for ACTN2 and ACTN3 in that they form a significant level of heterodimers in vivo (aligning 1234 to 4'3'2'1'). This affects interpretation of R577x because it lies within SP3 of ACTN3 -- even if a transcript were still made that evaded nonsense-mediated decay, no dimer would be expected to form from non co-evolved SP1 and SP2. | |||
This structure induces important paired co-evolutionary constraints. For example, SP3 cannot fix a mutational change that might be acceptable in terms of its standalone fold unless this change is also compatible with its binding surface with SP2, yet a near-neutral slightly adverse change could be followed by compensatory change in SP2 (respectively SP2'). | |||
To the extent ACTN2 and ACTN3 form both homo- and heterodimers in a phenotypically significant manner, there are both internal and external co-evolutionary constraints on each spectrin binding patch residue. The default evolutionary hypothesis here is a segmental gene duplication of ACTN2 occured creating ACTN3, raising gene dosage and introducing heterodimers (initially of identical sequence). Over time, protein sequences diverged with some retention of significantly different but still co-evolutionarily constrained heterodimers, accompanied by a degree of regulatory divergence in developmental timing and cellular location of expression. | |||
At the domain scale, should a spectrin repeat be lost in one allele through deletion or alternative splicing, say the third, that creates the problem of aligning 124 to 4321 from the remaining wildtype allele (all actinins lie on autosomal genes) or more subtly 124 to 421 which mis-pairs SP2 to itself whereas it was evolutionary adapted to compliment SP3. | |||
Thus it comes as no surprise to find all vertebrate actinins retain four spectrin subunits over tens of billions of years of branch time (see reference sequences). The apparent exception -- loss of SP4 in ACTN4 in human -- is due to annotation error at SwissProt and pipeline databases, the domain is in fact present though diverged from canonical sequence. | |||
(more to be added shortly) | |||
Revision as of 15:53, 10 December 2008
Introduction to ACTN3 comparative genomics
The alpha actinin gene ACTN3 is a coding gene on human chromosome 11, quite interesting in its own right but best known as ground zero in the debate over frivolity and unexpected consequences of personal genomics. This gene first of all needs careful and exhaustive re-annotation before considering this controversy because its existing peer-reviewed scientific literature (some 22 papers) is a mixture of pre-genomic era obsolescence and gross factual errors such as expression said specific to skeletal muscle.
Some unfortunate historic terminology needs to be explained. Actinins were erroneously thought similar to actin in early studies of myofibrillar components; instead they are homologically unrelated proteins that happen to bind actin. These 'actinins' were then improperly divided into 3 classes (alpha, beta, gamma) before it became known that their respective gene families were wholly unrelated (not homologous). For example, 'beta' actinins refer to heterodimers functioning as actin barbed-end capping proteins in skeletal muscle; they are comprised of the distinct gene families CAPZA and CAPZB themselves non-homologous and thus further misnamed. 'Gamma' actinins refer to yet other unrelated genes.
In this article, actinin shall mean alpha actinin, ie a protein encoded by one of the four paralogous genes ACTN1-ACTN4. Gene names are used for both gene or gene product (as this is always clear from context). Genus and species are indicated with standard 6-letter code (eg ACTN3_homSap). Care still must be taken with published articles and genBank entries that may fail to specify the alpha actinin under consideration despite a 2003 article calling for adherence to HGNC international nomenclature standards followed here.
The comparative genomics situation is further confused by high sequence conservation within the ACTN gene family, by paralog loss in some clades, by possible independent duplication events, and by pre-duplication parental genes only in early deuterostomes. It is not easy to assign transcripts or genomic fragments to correct orthology class by methods such as best reciprocal Blast, especially when the query itself is a fragment (eg third spectrin domain of ACTN3). Many genBank entries are unlabelled, mislabelled, or ambiguously labelled as to correct ortholog family.
However a reliable actinin classifier can be built by requiring flanking gene synteny, diagnostic signature residues and indels in building the reference sequence seed collection that focus on signature regions in which ortholog classes differ significantly from each other. For example ACTN2/3 share a five-residue deletion in exon 19 relative to (ancestral-length) ACTN1/4.
The human ortholog of ACTN3 is unusual (but not unique) in having a fairly abundant null allele, R577x, meaning the arginine at position 577 of the 901 residue protein has been replaced by a stop codon. Worldwide 18% of the human population is homozygous 577x 577x. It is very unlikely that a functional truncated protein can be produced because even if the promoter region is still functional, the mRNA would be degraded by nonsense-mediated decay (with no possibility of selenocysteine substitution) and the stable quaternary dimer necessary for function cannot form (as explained below).
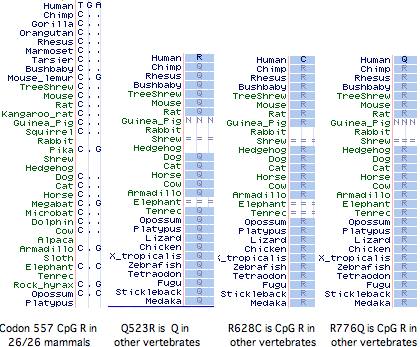
It has not been established whether the 577x was the initial inactivating mutation, as 3 additional amino acid changes (Q523R, R628C, R776Q) have also accrued at otherwise invariant sites in this allele (ie, in the dna donor to the public human genome relative to genBank reference sequence NM_001104). The latter two substitutions are also CpG mutational hotspots (the entire mRNA has 131 such sites). It is not known whether these other changes became widespread before or after R577x nor whether they affect ACTN3 function. However it is not easy to inactivate a large structural protein comprised of independent modules by single substitutions.
Curiously Q523R, R577x, and R628C all occur in the third spectrin repeat despite this region constituting only 11% of the gene, yet not a single nonsynonymous base change has occured in the 2706 bp coding region. With the advent of HapMap and similar projects, the phenotypic associations of these changes, possible co-occurence with wildtype R577, and the date(s) of 577x founder mutations could be resolved.
All mammals with assembled genomes encode a CpG hotspot at codon 577. This has transitioned to TpG in the human 577x allele but is not a polymorphic site in any other known mammal, though the search has been restricted to the individual animals used in genome projects (since transcripts rarely extend this far), plus 36 unrelated baboons and 33 chimpanzees all genotyping to ‘wild-type’ 577R. Thus there is no support for 577x as balanced polymorphism in any mammal other than human even though Z-line skeletal muscle structures may be very similar.
In regards to the supposed evolutionary advantage of various allele combinations (proxied by sprinting or endurance sports prowess), humans remain slow and weak relative to other mammals irregardless of their codon 577 status. The fastest human sprinter cannot outrun a dog with heartworms, much les a rabbit or chubby grizzly bear. A wild male chimp -- without any training or drug enhancement -- has the strength and aggression to rip apart the fittest human cage fighter.
Complete loss of ACTN3 does not give rise to a disease state or even observable phenotype in humans, despite a fallacious initial association with dystrophinopathy. Double knockouts of the orthologous gene in mouse are quite viable but exhibit various measurable effects. Over evolutionary time, the gene has been lost by natural genomic deletion in chicken and finch without known impact, yet retained in lizard, snake, and frog and even doubled in zebrafish (but not other rayfinned fish), again without known effect.
This suggested to some that human ACTN3 had an inessential or inconsequential physiological role to begin with (or become so since divergence with chimps), with its loss readiliy compensatable by other genes (presumably the 80% paralog ACTN3 with which it forms a heterodimer). In this view, ACTN3 may be on its way out the door, to disappear over time as a biallelic pseudogene. Given that over 80 other human genes have been lost, some very conserved over long evolutionary spans] since chimp divergence, it would not be surprising to catch a loss-in-progress.
The primary hurdle to clear here is the extraordinary conservation (proteome: 90th percentile) over 450 million years of ACTN3 amino acid sequence. It appears that this gene arose by segmental duplication from ancestral ACTN2 after the divergence of chondrichthyes (where all matches are ambiguous with respect to ACTN2/3). Gene duplicates are not usually retained over such a time frame unless they have a distinct functional niche that provides selective protection from constantly accruing deleterious mutations ('use it or lose it').
It is quite possible that earlier loss of a companion gene essential to the functional chain of ACTN3 has triggered its subsequent degeneration. The skeletal myosin gene MYH16 is potentially an attractive candidate, though actinins do not bind myosins directly but rather via actin. If ACTN3 functionality overlapped with ACTN2 apart from a critical role involving MYH16, then the loss of the latter gene would leave ACTN2 with no role that could not be compensated for by ACTN3. MYH16 was also lost to an internal stop codon after a half-billiion years of conservation following its separation from other myosins.
Another explanation is balanced polymorphism ('somewhat less is more') along the lines of allele proportions maintained in sickle-cell hemoglobins (heterozygosity can be selectively advantageous in malarial resistance). The idea here that human groups are benefited if some individuals have exceptional speed or endurance.
For ACTN3, frequencies of the three possible diploid states (R577/R577, R577/577x, 577x/R577x) vary by ethnic group and supposedly correlate -- imperfectly but predictively -- with athletic prowess. While single-locus genetic determinism is preposterous as determinative of a complex and vaguely specified phenotype, this has nonetheless gained traction in the popular mindset.
Correlations per se have a poor track record in establishing causality -- for example, a low IQ might also correlate quite strongly with interest and participation in sports. Perhaps R577x (ACTN3 is in fact expressed in brain) merely contributes to low IQ.
Phenotypic effects of ACTN1, ACTN2, and ACTN4 loss
Total absence of ACTN3 in human and double knockout mice does not result in genetic disease. What about loss of its three paralogs?
ACTN4 is only member of the actinin gene family mapped to a human disease, focal segmental glomerulosclerosis, FSGS1, autosomal dominant toxic gain-of-function when any of 5 distinct substitutions (eg W59R, I149del, K255E, R310Q and V801) cause it to bind too tightly (persistent switched on mode) to the actin cytoskeleton in renal glomerular podocytes (visceral epithelial cells of kidneys involved in filtration). This eventually leads to renal failure.
ACTN4 however is also widely expressed with a variety of roles (motility, adhesion, endocytosis). Its two amino-terminal calponin homology domains crosslink actin filaments (F-actin) as regulated by Ca2+. Certain substitutions divert its usual localization away from actin stress fibers and focal adhesions. The question is whether paralogous mutations in the calponin homology domains of other actinins would have comparable effects. That could be studied in knock-in mouse ACTN3 to help determine its normal functions.
The structure of the K255E protein shows that the calponin domains remain in compact configuration despite disruption of the inter-calponin bridge to Trp147 of CH1.
Less is known about disease alleles of ACTN2. A single report of dilated cardiomyopathy (CMD) seemingly attributably to a heterozygous missense mutation at a conserved residue
1. Mohapatra, Jimenez Lin Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Molec. Genet. Metab. 80: 207-215, 2003.
ACTN3 transcription is NOT restricted to skeletal muscle
In dozens of articles in low-impact factor journals and on at least one personal genomics advice page, it has been implied that ACTN3 is expressed exclusively in type 2 skeletal muscle fibers at the Z-line and so its absence or partial replacement must consequently relate to fast or slow twitch fiber performance. These copycat cites track back to a 1992 paper (see M86407 'skeletal muscle specific' 30-OCT-1994).
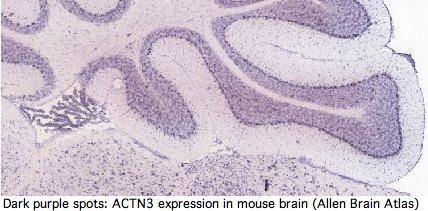
Yet GenBank and transcript-detecting chips contain numerous validated ACTN3 transcripts from other tissues, including heart muscle, pericardium, prostate, medulla oblongata, brain, salivary gland, skin, eye, hematopoietic stem cells, gastrointestinal tract, and testis. From its assembly, it can be seen that tree shrew genome contains a full length processed pseudogene -- which requires high expression of ACTN3 in germline tissue.
Actinins make cytoskeleton sense in all these cell types but none contain skeletal muscle fiber. It follows that ACTN3 has other functions unless the dominant modes of expression of this ancient oonserved gene are gratuitous. Heart muscle obviously has a role in athletics but it is not of skeletal muscle type. The transcript database GeneHub-GEPIS shows heart with markedly higher expression of ACTN3 than skeletal muscle. Testis and hematopoietic cells too evidently play a role in athletic performance (why else would Tour de France cyclists load up on testosterone patches and erythropoietin shots?).
The interpretation of R577x has largely been based on the 'plausibility' of its expression in skeletal muscle explaining performance correlation. The argument largely vaporizes because ACTN3 is not expressed exclusively in skeletal muscle. It is just as easy to spin a tale of how a role in heart muscle or testes might account for the observations. Perhaps ACTN2 can compensate in skeletal muscle but not elsewhere. Thus the burden of proof requires demonstrating that ACTN3 roles in non-skeletal tissues do not have more explanatory power.
Thus ACTN3 is a good gene for illustrating the problems accompaning personal genomics testing. Some people will make significant life decisions based on test results irregardless of disclaimers. If the accompaning explanation is shoddy -- and ACNT3 is commonly explained at the intellectual depth of an astrology column -- they cannot act in an informed way. At a bare minimum, ACTN3 needs a very detailed review of its published literature and comparative genomics. The widespread error in describing ACTN3 expression specificity is only the beginning of the serious omissions in this gene's existing annotation.
It is an immense public disservice to inform the public there is "a gene for college athletic scholarships" so their potentially elite 6-year old should be sequenced at R577 and channeled into proper coaching early on. In reality, hundreds of genes (not to mention environmental factors) are involved in vague phenotypes. A given gene may exhibit a statisically significant effect yet have very little predictive power.
ACTN3 transcription is NOT restricted to skeletal muscle: DN887432 Rabbit eye minus lens and cornea. AK313556 Homo sapiens cDNA pericardium DA827389 Homo sapiens cDNA clone PERIC2008976 pericardium BP318079 Homo sapiens cDNA Sugano cDNA library, pericardium AK125851 Homo sapiens cDNA testis AK303044 Homo sapiens cDNA clone TESTI4007778 testis GeneHub-GEPIS: human ACTN3 expressed in GI tract, heart, muscle, testis GeneHub-GEPIS: mouse ACTN3 expressed in bone, brain muscle, salivary gland, skin AK134757 Mus musculus adult male medulla oblongata cDNA, RIKEN full-length DT909761 Mus musculus cDNA Hematopoietic stem cells AF093775 Mus musculus alpha-actinin 3 (Actn3) mRNA, complete cds. BC111890 Mus musculus actinin alpha 3, mRNA (cDNA clone BC166600 Rattus norvegicus actinin alpha 3 mRNA Prostate
Phenotypic effects of ACTN1, ACTN2, and ACTN4 loss
Total absence of ACTN3, a well-conserved gene, in human and double knockout mice does not result in genetic disease. What about loss of its three paralogs?
ACTN4 is only member of the actinin gene family mapped to a human disease, focal segmental glomerulosclerosis, FSGS1. However no known mutation results in gene loss (which may be embryonic-lethal). Instead, all are autosomal dominant gain-of-function. Any of 6 distinct substitutions W59R, I149del, K255E, S262F, R310Q and V801 cause ACTN4 to bind too tightly (persistent switched-on mode) to the actin cytoskeleton in renal glomerular podocytes (visceral epithelial cells of kidneys involved in macromolecular filtration), resulting in a condition eventually progressing to renal failure. The mouse model for K255E is comparable.
Its tandem amino terminal calponin-homology CH domains crosslink actin filaments as regulated by Ca2+. These substitutions divert its usual localization away from actin stress fibers and focal adhesion points. As ACTN4 is also widely expressed in a variety of other cell types with diverse roles (motility, adhesion, endocytosis, tight junctions), this suggests pleiotropic affects beyond podocyte dysfunction.
ACTN4 forms a homodimer, meaning a compensation scenario as in ACTN2 replacement of ACTN3 is not feasible. The question is whether paralogous mutations in the calponin homology domains of other actinins have similar effects. That could be studied in knock-in mouse ACTN3 to help determine its normal functions. Sufficient conservation makes this feasible. The structure of the K255E protein shows that the calponin domains remain in compact configuration despite disruption of the CH2 bridge to W147 of CH1.
Less is known about disease alleles of ACTN2. A single report of dilated cardiomyopathy (CMD) seemingly attributs it to a heterozygous missense mutation at a conserved residue Q9R (which significantly precedes CH1). Again an autosomal dominant effect rather than loss, this mutation affected normal interaction with muscle LIM protein (MLP) and nuclear localization. The effect in ACTN3 of a paralogous mutation is not known.
No disease allele of ACTN1 has ever been reported. This in itself is not surprising since only 1,500 human genes out of 20,000 (7.5%) have known disease alleles. However it could reflect essential functionality. ACTN1 does have a novel activity for an actinin, binding the metabotropic glutamate receptor type 5b, a GPCR protein rather than an ion channel, modulating receptor cell surface expression. An induced tyrosine phosphorylation mutation Y12F in pressure-induced adhesion response has been investigated. Despite oft-repeated claims to the contrary, ACTN1 is in fact expressed in skeletal muscle.
R577x as ACTN3 phyloSNP
An alignment of exon 15 (which comprises about half of the third spectrin domain) shows that codon 577 was ancestrally K (lysine) in early vertebrates, a residue persisting without exception to the present day in all extant vertebrates diverging before ornAna (platypus). In the mammalian stem, K was replaced by R (arginine) and that residue persisted in mammals (26/26 species). This is the definition of phyloSNP -- a clade-defining synapomorphy with persistent ancestral state whose conservation both before and after implies structural and/or functional significance in both the clade and its complementary tree but different roles. Evidently residue 577 has long been significant in the third spectrin domain of ACTN3, implying about any change in humans is disadvantageous.
The non-mammalian sequences below had to be individually established as orthologs of the human ACTN3 gene using retained flanking synteny of neighboring genes because the UCSC comparative genomics track misaligns paralogs here. The retained synteny is not always two-sided and in some cases inversions have resulted in loss of immediate adjacency.
R577x and co-evolution of actinin spectrin repeats
The four alpha actinin paralogs in human all have the same domain structure, namely 2 calponin-homology domains (that bind actin) followed by 4 spectrin rod domains followed by 2 EF hands that ancestrally bound calcium. These domains comprise almost the entirety of the 901 amino acids of ACTN3. All three domain types are exceedingly common in hundreds of human proteins in various permutations, associations, and repeat numbers.
Actinins are a classic chimeric domain protein. The roles of each domain types are fairly well-known and excellent 3D domain structures are available; as an approximation, these domain folds independently.Actinins form anti-parallel dimers via their spectrin domains, meaning the overall shape is that of a dumbbell. Dimerization (ie two binding sites) is essential to the basic actin cross-linking functionality of actinins. ACTN3 and ACTN2 can form heterodimers. Related proteins such as dystrophin with similiar chains of spectrin domains could conceivably interact with actinins in a similar manner.
In many domain-shuffled proteins (eg titin), the number of internal repeats is both evolutionarily unstable (due to the intrinsic propensity for mis-aligned recombination or replication slippage) and subject to domain-skipping alternative splicing, seemingly without drastic end-functional consequences. Such chimeric proteins are notoriously difficult to align homologically (ie by evolutionary descent) because quite different histories of contraction and expansion can result in same domain copy position.
Here it must be noted that splice boundaries in domain-shuffled proteins (21 coding exons in all actinins) do not necessarily allow for clean excision of domains, apparently depending on the relative timing of protein origin relative to the main era of intron gain. It appears that the ancestral actinin gene (with its internal domain repeat expansion complete) arose very early, prior to exonization, whereas its segmental gene duplications to vertebrate paralogs took place much later, subsequent to exonization, yet not so recently that any flanking gene synteny nor any sequence alignability across spectrin domains could be retained.
When actinins from early eukaryotes are considered, it emerges that SP1 is likely the ancestral spectrin domain (one such domain still suffices for dimerization). An internal duplication prior to fungal divergence gave rise to SP4 and a protein with two spectrin repeats. SP2 and SP3 apparently arose from a second intragenic divergence, giving rise to the four spectin repeats seen in all extant vertebrate actinins.
The number of repeats determine the length of spacer, total binding strength, and access to other binding proteins such as fessilin. Note the spectrin gene SPTA1 contains 22 spectrin domains, so the 4 of actinins is not exceptional.
The domain composition of ACTN3 is shown relative to its exons below, followed by a comparison of ACTN gene family chromosomal contexts.
>ACTN3_homSap Homo sapiens (human) 21 exons 901aa NM_001104 Q523R R577x R628C R776Q CH1 Ch2 SP1 SP2 SP3 SP4 EF1 <font color="#999900"EF2 0 MMMVMQPEGLGAGEGRFAGGGGGGEYMEQEEDWDRDLLLDPAWEKQQRK 0 0 TFTAWCNSHLRKAGTQIENIEEDFRNGLKLMLLLEVIS 1 2 GERLPRPDKGKMRFHKIANVNKALDFIASKGVKLVSIGAE 1 2 EIVDGNLKMTLGMIWTIILRFAIQDISVE 1 2 ETSAKEGLLLWCQRKTAPYRNVNVQNFHTS 2 1 WKDGLALCALIHRHRPDLIDYAKLR 0 0 KDDPIGNLNTAFEVAEKYLDIPKMLDAE 1 2 DIVNTPKPDEKAIMTYVSCFYHAFAGAEQ 0 0 AETAANRICKVLAVNQENEKLMEEYEKLASE 0 0 LLEWIRRTVPWLENRVGEPSMSAMQRKLEDFRDYRRLHKPPRIQEKCQLEINFNTLQTKLRLSHRPAFMPSEGKLVS 0 0 DIANAWRGLEQVEKGYEDWLLSEIRRLQRLQHLAEKFRQKASLHEAWTR 1 2 GKEEMLSQRDYDSALLQEVRALLRRHEAFESDLAAHQDRVEHIAALAQELN 2 1 ELDYHEAASVNSRCQAICDQWDNLGTLTQKRRDALE 0 0 RMEKLLETIDQLQLEFARRAAPFNNWLDGAVEDLQDVWLVHSVEETQ 0 0 SLLTAHDQFKATLPEADRERGAIMGIQGEIQKICQTYGLRPCSTNPYITLSPQDINTKWDM 0 0 VRKLVPSRDQTLQEELARQQVNERLRRQFAAQANAIGPWIQAKVE 0 0 EVGRLAAGLAGSLEEQMAGLRQQEQNIINYKTNIDRLEGDHQLLQESLVFDNKHTVYSME 0 0 HIRVGWEQLLTSIARTINEVENQVLTRDAKGLSQEQLNEFRASFNHFDR 0 0 KRNGMMEPDDFRACLISMGYDL 0 0 GEVEFARIMTMVDPNAAGVVTFQAFIDFMTRETAETDTTEQVVASFKILAGDK 0 0 NYITPEELRRELPAKQAEYCIRRMVPYKGSGAPAGALDYVAFSSALYGESDL* 0
Spectrin repeats (independently folding rodlike structural spacers without catalytic, post-translational modifications, regulatory substrate, or non-homlogous protein binding sites) might seem especially subject to these problems. However that cannot be not the case in actinins because the proteins invariably occur as dimers joined along the spectrin interface. This forces a two-fold symmetry axis per standard theories of protein oligomericity (fully supported by crytallographic studies), meaning anti-parallel association of spectrin domains.
In other words, SP1 of one actinin monomer binds to SP4 of the other and SP2 to SP3 (ie 1234 to 4321). That has an additional twist for ACTN2 and ACTN3 in that they form a significant level of heterodimers in vivo (aligning 1234 to 4'3'2'1'). This affects interpretation of R577x because it lies within SP3 of ACTN3 -- even if a transcript were still made that evaded nonsense-mediated decay, no dimer would be expected to form from non co-evolved SP1 and SP2.
This structure induces important paired co-evolutionary constraints. For example, SP3 cannot fix a mutational change that might be acceptable in terms of its standalone fold unless this change is also compatible with its binding surface with SP2, yet a near-neutral slightly adverse change could be followed by compensatory change in SP2 (respectively SP2').
To the extent ACTN2 and ACTN3 form both homo- and heterodimers in a phenotypically significant manner, there are both internal and external co-evolutionary constraints on each spectrin binding patch residue. The default evolutionary hypothesis here is a segmental gene duplication of ACTN2 occured creating ACTN3, raising gene dosage and introducing heterodimers (initially of identical sequence). Over time, protein sequences diverged with some retention of significantly different but still co-evolutionarily constrained heterodimers, accompanied by a degree of regulatory divergence in developmental timing and cellular location of expression.
At the domain scale, should a spectrin repeat be lost in one allele through deletion or alternative splicing, say the third, that creates the problem of aligning 124 to 4321 from the remaining wildtype allele (all actinins lie on autosomal genes) or more subtly 124 to 421 which mis-pairs SP2 to itself whereas it was evolutionary adapted to compliment SP3.
Thus it comes as no surprise to find all vertebrate actinins retain four spectrin subunits over tens of billions of years of branch time (see reference sequences). The apparent exception -- loss of SP4 in ACTN4 in human -- is due to annotation error at SwissProt and pipeline databases, the domain is in fact present though diverged from canonical sequence. (more to be added shortly)