Selenoprotein evolution: GPX: Difference between revisions
Tomemerald (talk | contribs) No edit summary |
Tomemerald (talk | contribs) No edit summary |
||
| Line 9: | Line 9: | ||
Note the three large deletions in GPX4, GPX7, and GPX8 relative to other GPX. The latter have ancestral length judging by the GPX4-classifying sequence in the metazoan outgroup species Monosiga brevicollis. Parsimonously, the deletions occured once in the common ancestral sequence to GPX4 and GPX7/8. It is already present in the tubeworm metazona Ridgeia. Thus the pattern of indels suggests grouping GPX4 with GPX7 and GPX8 to the exclusion of GPX1 and GPX2. | Note the three large deletions in GPX4, GPX7, and GPX8 relative to other GPX. The latter have ancestral length judging by the GPX4-classifying sequence in the metazoan outgroup species Monosiga brevicollis. Parsimonously, the deletions occured once in the common ancestral sequence to GPX4 and GPX7/8. It is already present in the tubeworm metazona Ridgeia. Thus the pattern of indels suggests grouping GPX4 with GPX7 and GPX8 to the exclusion of GPX1 and GPX2. | ||
It can be seen that most cysteines occur sporadically or just within a particular paralog group, the exceptions being the univeral selenocysteine site (occupied by cysteine in some paralog families) and a following cysteine 30 residues distal (eg | <br clear="all" /> | ||
[[Image:GPX keyRes.jpg|left|]] | |||
It can be seen that most proteinwide cysteines occur sporadically or align just within a particular paralog group, the exceptions being the univeral selenocysteine site (occupied by cysteine in some paralog families and serine anomalously in teleost fish) and a following cysteine 30 residues distal (eg UGKTEVNYTQLVDLHARYAECGLRILAFPC in GPX4_homSap). These two residues very likely form a mixed diselenide (resp. disulfide) with an essential role in the redox reactions carried out by glutathione peroxidases. However some exceptions occur in CPX3 and GPX2. | |||
Other cysteines might be in structural disulfides yet in some GPX an odd number occur, meaning they could not all pair off. This, in conjunction with non-homologous positions, argues against structural disulfides in most cases. | |||
Note GPX7 and GPX8 have classical KDEL endoplasmic reticulum retention signals. This implies an interaction with the protein systems responsible for retrograde translocation and retention. This subcellular localization apparently arose in GPX7 post-amphioxus divergence since the motif is missing there, in sea urchin, and early eukaryotes. GPX8 arose as a subsequent duplication of this ancestral GPX7 and so inherited the motif. | Note GPX7 and GPX8 have classical KDEL endoplasmic reticulum retention signals. This implies an interaction with the protein systems responsible for retrograde translocation and retention. This subcellular localization apparently arose in GPX7 post-amphioxus divergence since the motif is missing there, in sea urchin, and early eukaryotes. GPX8 arose as a subsequent duplication of this ancestral GPX7 and so inherited the motif. | ||
Revision as of 14:25, 27 May 2008
The GPX family of proteins can be traced back to great phylogenetic depth. It has experienced various expansions, some narrowly lineage-specific but others retained very broadly. Gene family contractions are more difficult to establish since absence of evidence in 2x genomic assemblies rarely constitutes evidence of absence. In vertebrates, the most notable recent expansion is of GPX3 in placental mammals into the divergently tandem pair GPX7 and GPX8. Marsupials and earlier diverging species lack these latter genes while retaining GPX3 in syntenically established orthologous position (flanking genes TNIP1 and ANXA6).
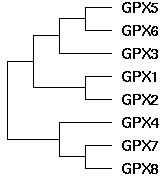
The topology of the GPX gene tree can be unambiguously established as ((((GPX5,GPX6),GPX3),(GPX1,GPX2)),(GPX4,(GPX7,GPX8))) using blastp clustering, chromosomal locations, rare genomic events such as indels, signature residues, and intron positions and phases. The main technical issue is the placement of GPX4, which can be resolved using the large indels it shares with GPX7 and GPX8 in addition to its sequence clustering. GPX4 is by far the most conserved member of this gene family, suggesting its function has not strayed far from its role in ancestral metazoa, even as its gene duplicates have subfunctionalized and sublocalized.
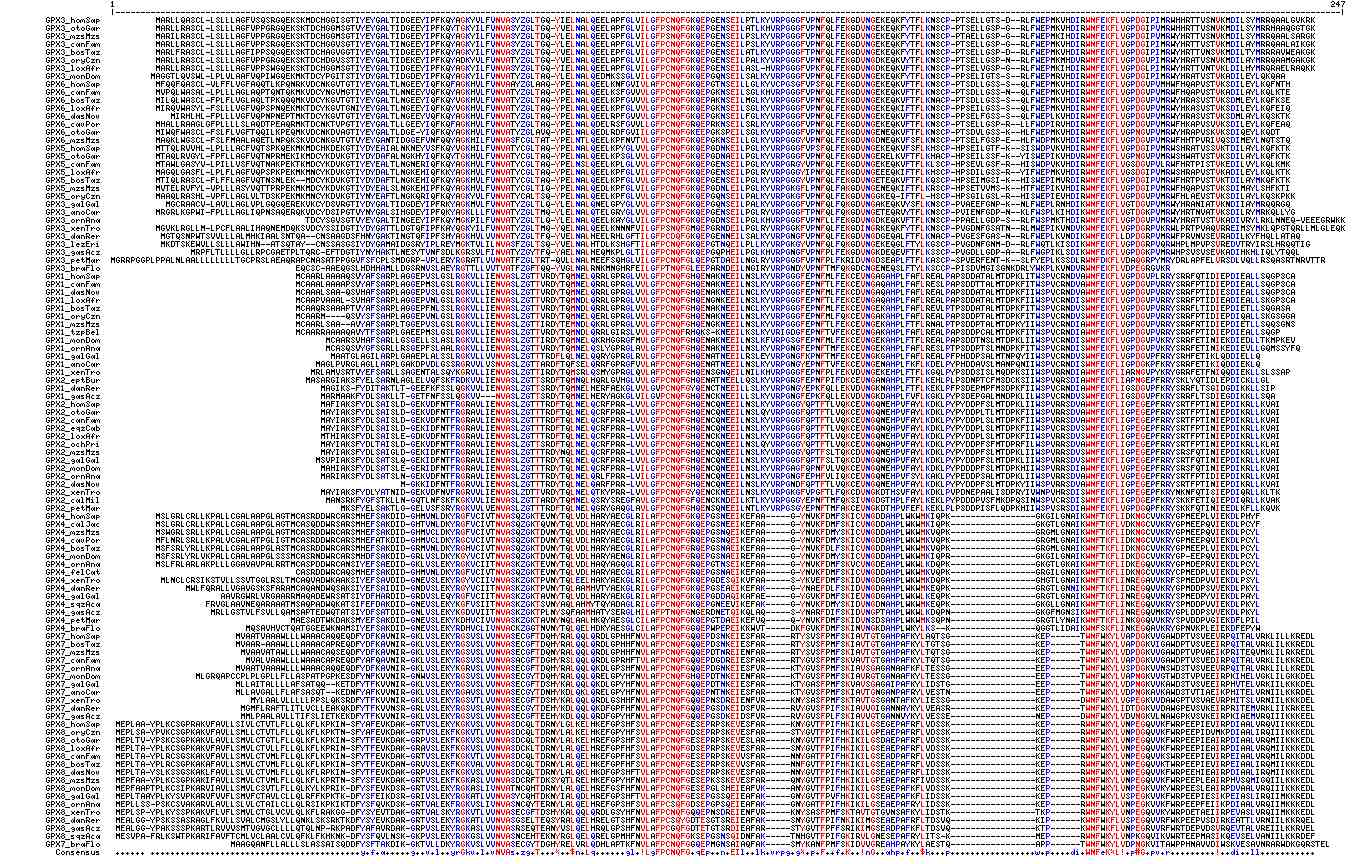
The alignment shows 103 of the 170 available GPX sequences evenly distributed (more or less) over both the eight members of the gene tree and the chordate species tree. The reddish color shows residues conserved at the 90% level; the bluish less-conserved at 70%. The selenocysteine is represented by Z because U is not etained by the alignment tool used here.
Note the three large deletions in GPX4, GPX7, and GPX8 relative to other GPX. The latter have ancestral length judging by the GPX4-classifying sequence in the metazoan outgroup species Monosiga brevicollis. Parsimonously, the deletions occured once in the common ancestral sequence to GPX4 and GPX7/8. It is already present in the tubeworm metazona Ridgeia. Thus the pattern of indels suggests grouping GPX4 with GPX7 and GPX8 to the exclusion of GPX1 and GPX2.
It can be seen that most proteinwide cysteines occur sporadically or align just within a particular paralog group, the exceptions being the univeral selenocysteine site (occupied by cysteine in some paralog families and serine anomalously in teleost fish) and a following cysteine 30 residues distal (eg UGKTEVNYTQLVDLHARYAECGLRILAFPC in GPX4_homSap). These two residues very likely form a mixed diselenide (resp. disulfide) with an essential role in the redox reactions carried out by glutathione peroxidases. However some exceptions occur in CPX3 and GPX2.
Other cysteines might be in structural disulfides yet in some GPX an odd number occur, meaning they could not all pair off. This, in conjunction with non-homologous positions, argues against structural disulfides in most cases.
Note GPX7 and GPX8 have classical KDEL endoplasmic reticulum retention signals. This implies an interaction with the protein systems responsible for retrograde translocation and retention. This subcellular localization apparently arose in GPX7 post-amphioxus divergence since the motif is missing there, in sea urchin, and early eukaryotes. GPX8 arose as a subsequent duplication of this ancestral GPX7 and so inherited the motif.