USH2A SNPs
Normal function of usherin
A dozen recent papers on hearing and vision have illuminated the normal function USH2A gene product via its binding with other domains and proteins and related diseases, important progress but still not sufficient to explain how and whether specific fibronectin variants lead to disease phenotypes.
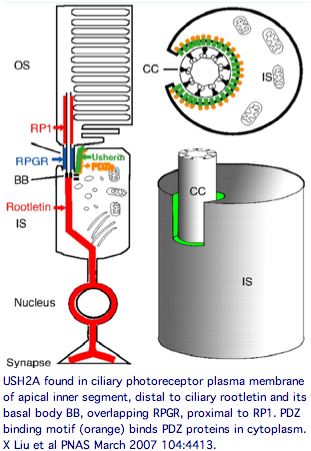
Protein binding partners of USH2A are important to identify because surface contacts in hetero-oligomers co-evolve and so have explanatory potential for point mutation and variation in USH2A. Prime suspects include gene products themselves causing vision and hearing disorders. The gene LCA5, responsible for Leber congenital amaurosis V, encodes lebercilin, a 697 residue protein involved in centrosomal and cilia. Lebercilin and usherin do not interact directly but rather via NLP encoded ninein-like protein at the basal bodies of the photoreceptor connecting cilia.
Both lebercilin and ninein-like have extended coiled-coil domains in addition to SMC (an ancient centrosomal chromosome segregation domain) and EF-hands (in NLP). NLP has not surfaced to date in vision disorders, perhaps because of an essential role in mitosis, a notation somewhat at variance with its moderate rate of divergence. Alternative alleles of NLP -- and indeed LCA5 through chain transitivity of binding effects -- could compensate or exacerbate pathogenic alleles of USH2A.
Coiled-coil helices could plausibly bind to an established groove on USH2A fibronectin domains though this has not been established as the NLP interaction mode. USH2A also has laminin, EGF and PDZ1 domains which commonly interact with other proteins.
Laminin domains of usherin, residues 518-1052 in short form numbering, bind the 7S domain of type IV collagen, with USH2B mutations in loop b but not loop d abolishing this binding. These experiments need to be revisited using full length usherin.
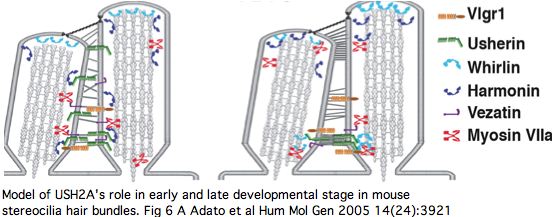
The PDZ1-binding domain at the usherin C-terminus (cytoplasmic side) bind both whirlin (DFNB31) and the scaffold protein harmonin (causative for USH1C) at synaptic terminals of both retinal photoreceptors and inner ear hair cells (and transiently during stereocilia development). Cell adhesion molecules in pre- and post-synaptic membranes keep synaptic clefts in proper register. Harmonin plays an integrative role across numerous proteins involved in type 1 Usher diseases, so this linkage to USH2A (and VLGR1 of USH2C) begins to explain how mutations in so many genes can give rise to quite similar disorders.
Usherin has a very specific localization within retinal rod cells (and is not expressed at ribbon synapses) a location that clarifies the role of its fibronectin domains to a certain extent. USH2A localizes to the plasma membrane of inner segments (but not that of the non-motile cilia), internally with its PDZ-motifs presumably anchored to the various PDZ proteins mentioned above and externally with laminin and fibronectin domains surrounding the cilia that connects to the rhodopsin-containing outer segment, perhaps spanning the 1000 angstrom wide band of periciliary matrix material between the plasma membranes of the inner segment and cilium. The physical proximity to a retinitis pigmentosa GTPase regulator gene RPGR (no associated deafness so not in Usher complex) is perhaps suggestive of an interaction but RPGR does not contain any domains that would bind to fibronectin.
The non-progressive deafness in USH2A can be explained by its transient expression in kinocilia. These, not the so-called sterocilia (actin filament-based membrane protrusions), are the structure related to the photoreceptor connecting cilia. Kinocilia resemble the membranous protrusions of the periciliary ridge complex of photoreceptors and could conceivably be homologous, recalling the many deep connections between the evolutionary origin of these sensory systems dating to cnidarian rhopalia.
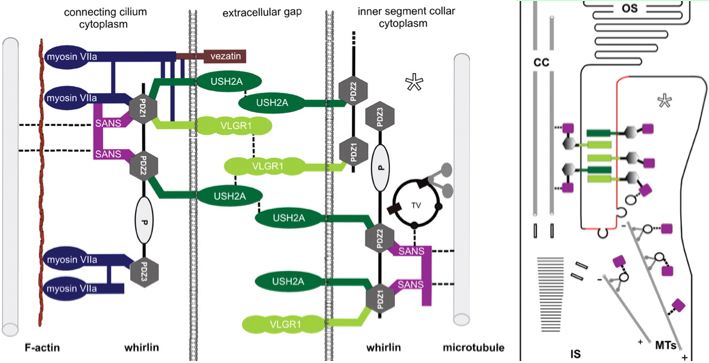
The DFNB31 gene product whirlin, USH2A and VLGR1 co-localize at the cilium and the outer membrane of photoreceptor cells as well as in spiral ganglion neurons of inner ear but this is related to PDZ domain binding in the cytoplasm, so does not illuminate extracellular fibronectin domains. However VLGR1 is also transmembrane and its ectodomain comprises some of the fibrous links connecting these adjacent membranes.
Vezatin, a ubiquitous adherens cell-cell junction protein whose primary interactions are with myosin VIIa via a FERM domain and the cadherin-catenins complex, also has a 2 transmembrane component. MYO7A (but not VEZT) has been implicated in Usher disorders. It has no recognized domains but a coiled-coil region 430..462 and transmembrane regions 139..159 and 162..182 have been predicted by ModBase and SwissProt so it is doubtful as a extracellular USH2A binding partner.
Keeping in mind that these proteins can expressed at many other sites in the organism, the overall retinal cytoplasmic Usher protein complex can be understood in the context of a reloading zone. Recall photoreceptor membranes in the outer segment turn over rapidly via RPE phagocytosis so require constant renewal of their components from the inner segment. This material is vectorially transported within the inner segment and then through the connecting cilium to the outer segment, requiring reloading in a specialized compartment of the apical inner segment.
The Usher protein complex in rod photoreceptor cells is usefully envisioned at left by T Maerker et al in Hum Mol Gen 2008 17(1):71. Protein interactions and vessicle transport in the ciliary/periciliary region. Ectodomains of USH2A and VLGR1 connect membranes of connecting cilium to inner segment. Whirlin PDZ domain anchors both in the cytoplasms. Dynein mediates vesicle transport along microtubules to the apical inner segment collar. Docking and fusion membrane sites are determined by the fixed Usher protein complex. Note USH2A does not actually reside on the cilium side and binding to VLGR1 and other USH2A chains (by unspecified domains) are speculative.
Note that fibronectin domains cannot merely serve as spacers with laminin domains doing the anchoring because the fibronectin domains are far too conserved. They cannot bind each other in anti-parallel fashion because all project out from same the inner segment side. VLGR1 is a very peculiar orphan GPCR with single pentraxin, LamGL and EPTP domains and many Calx-beta domains in its long N-terminal ectodomain (that don't seem to bind calcium); some of these resemble integrin-beta4 domains.
Of the known proteins in the Usher network, VLGR1 is the most promising in terms of perhaps having domains that bind and co-evolve with USH2A. But without a 3D structure of FN3 binding to some VLGR1 domain, the importance of surface residues in fibronectins is difficult to evaluate.
However the list of proteins in the Usher network has grown steadily in the last 2-3 years and perhaps other disease genes will add to the 38 relevent ones at RetNet. Indeed a recent photocilium proteomics report finds rather too many new genes, 1968 proteins in mouse rod outer segment, its cytoskeleton, axoneme, basal body, and ciliary rootlet.
These include 13 genes known to cause cilia disease and 7 intraflagellar transport proteins, though the bulk of the protein list comes from the outer segment (based on rootletin knockouts). Protein copy number gives some idea of relative stoichiometries based on 70 million rhodoposin molecules per mouse rod cell and GNAT1 transducin at a tenth that level.
By comparison, USH2A is present at 3,000 copies, CROCC (rootilin) at 220,000 copies, MYO7A at 1,000 copies and RP1 at 14,000 copies but oddly VLGR1, DFNB1 and VEZT were not detected.