Iron sulfur clusters
Introduction
The surprisingly numerous nuclear proteins containing 4Fe-4S clusters are made from their respective apoproteins in the cytoplasm during the final stages of an assembly process that begins within mitochondria and ends with an embedded cluster in polymerases, helicases, primases, telomerases, and photolyases with no explained need for a cofactor otherwise associated with oxidation and reduction.
These 4Fe-4S clusters do not spontaneously associate with their target protein because they do not occur in free solution, being quite unstable to unwanted oxidation. Instead, nascent clusters are attached to a series of mediating proteins, carrier scaffolds and conformational chaperones throughout a complex process of maturation. That process and the gene products involved -- which are conserved from yeast to human -- have been recently reviewed in depth and new results (1,2) have clarified the roles of the four main protein components that collaborate on the final stage of cytoplasmic assembly.
Not all extra-mitochondrial 4Fe-4S cluster proteins are assembled in this pathway, but the molecular basis for specificity has not yet been determined. Indeed, a surprising number of proteins -- some studied for decades -- have only been recognized as iron sulfur proteins in 2011-12.
Indeed, the list of proteins is still incomplete because many unrelated homology classes have Fe-S clusters, meaning no single diagnostic pattern can be used to scan the entire proteome. Often four conserved cysteines coordinate the cubane complex but their spacing within the primary sequence is not uniform and difficult to distinguish from cysteine patterns that bind intrinsic zinc. Further confusing matters, seemingly artifactual zinc can replace bona fide 4Fe-4S clusters in proteins purified for crystallography in the presence of oxygen (1,2,3).
The early and middle stages of intra-mitochondrial iron sulfur cluster assembly are carried out by gene products of bacterial origin, relics of alphaproteobacterial endosymbiosis transferred long ago to the nuclear genome. However, not all components of final cytoplasmic assembly have such a clear origin whereas most targeted apoproteins (such as primase large subunit PRIM2) are clearly those of the archaeal parent. Thus the final stage of assembly presents two worlds in collision: bacterial proteins assembly iron sulfur clusters in unfamiliar archaeal proteins.
Bioinformatics, while a poor substitute for experimentation, is fast and easy, so it is best to exhaust the possibilities there first. Nothing is proven but sometimes it can suggest interesting directions.
MMS19: a large all-scaffold protein
MMS19 is a large protein involved in cytoplasmic iron sulfur assembly first studied with bioinformatic tools 12 years ago (1,2). Revisiting that with modern comparative genomics methods, MMS19 emerges as a modular scaffolding protein over its entire length, conserved in its features -- though not particularly in amino acid sequence -- from the earliest diverging eukaryotes to human.
The C-terminus of MMS19 was initially classified as HEAT repeats. Today we know these are not found as individual units but instead work together to form a long twisted spiral of consecutive modules called an ARM domain. An individual HEAT unit consists of a small 3-helix bundle, a generic super-secondary structure analogous to a beta-alpha-beta Rossmann fold unit, meaning most occurrences of HEAT in the eukaryotic proteome are not truly homologous despite structural similarity but instead represent convergent evolution analogous to Rossmann-like fold units forming many unrelated beta propellers or TIM barrels.
Since these domains are catalytically inert and lack conserved cysteins or other conserved motifs, MMS19 can contribute as organizing principle to the cytoplasmic iron assembly complex (and other nuclear complexes) but not to the actual business of forming 4Fe-4S on target apoproteins.
The size of MMS19 -- over a thousand residues -- makes it a difficult target for structure determination. As of June 2012, no deposited structure at PDB provides a template upon which the MMS19 can be threaded. In the interim, MMS19 might be structurally modeled using the known beta-catenin structure -- despite its lack of authentic homology, it too is comprised almost entirely of HEAT units.
The number of HEAT repeats in an ARM domain is subject to expansion and contraction over evolutionary time. The individual units often align poorly with each other and generally lack conserved residue signatures despite initial reports, yet this level of variation does not necessarily affect the overall fold. However, this lack of diagnostic features makes it difficult to reliably identify remote homologs of HEAT repeats because the primary sequences can be diverged beyond recognition and homological alignments go out of register when the number of repeats differs.
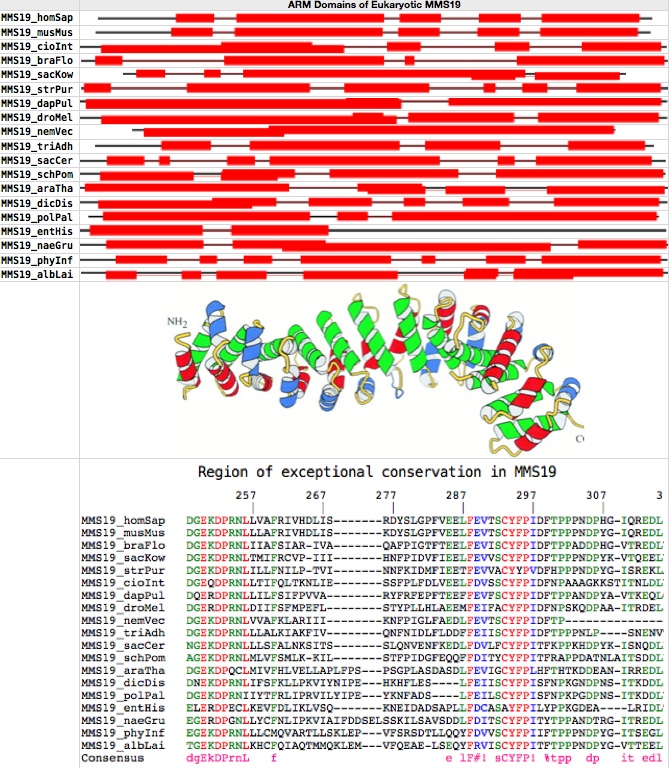
An accurate count of the number of individual HEAT domains in MMS19 from a given species is also difficult because some domains are more accurately represented in the HMMer profile than others, here giving different counts between mouse and human at UniProt despite 90% sequence identity over their entire length. Indeed the five species with manually reviewed UniProt entries are all in conflict, with the nominal number of HEAT units range from 7 in human to 18 in slime mold, despite similar lengths and overall alignment implying the same actual domain structure.
MMS19 is a single-copy gene without paralogs in all eukaryotes, implying simple orthology (no retained duplications or losses). Below, 20 full length fasta sequences from GenBank were chosen for uniform distribution over the eukaryotic phylogenetic tree. Superfamily proved to be the most consistent, sensitive and selective online tool for ARM domain detection. The figure at bottom establishes that MMS19 consists entirely of HEAT units and spacers, which in effect form a single ARM.
It emerges upon alignment with MultAlin that conservation is mediocre overall except for one previously notedspecial region of exceptional conservation containing two blocks of invariant residues from human to yeast to amoeba. This region must have already been established in the last common ancestor of all eukaryotes and play a very special role in MMS19 even today to account its conservation over trillions of years of cumulative branch length. However that role remains a complete mystery. Sequence conservation in MMS19 is otherwise not exceptional: typically 27-34% identity relative to human, of which some portion is accidental.
ultra-conserved region in MMS19: human: 182 DGEKDPRNLLVAFRIVHDLISRDYSLGPFVEELFEVTSCYFPIDFTPPPNDPHGIQREDL 241 yeast: 184 NGEKDPRNLLLSFALNKSITSSLQNVENFKEDLFDVLFCYFPITFKPPKHDPYKISNQDL 24
Regardless of Blast query -- full-length MMS19, this ultra-conserved region, or reconstructed ancestral sequence -- no counterpart to MMS19 occurs among 2,500 complete bacteria and archaea genomes, even though unambiguous orthologs to human MMS19 are readily found in the earliest diverging eukaryotes. MMS19 may thus represent a eukaryotic innovation needed to organize more complex cytoplasmic iron assembly, or be too simplified and diverged (or just lost) in prokaryotes. As method of last resort, prokaryotic operons containing other cytoplasmic iron sulfur assembly proteins could be scanned for adjacent HEAT-like domains or comparable scaffolding proteins.
There are no matches to MMS19 at PDB using Blastp. Since the fold is widespread and generic, structural matches in DALI do not imply homology. On the other hand, this allows the crystallographic structure of a non-homologous ARM protein (beta-catennin pdb: 1LUJ) to serve as provisional structural template. Bound E-cadherin, ICAT, XTCF3 complexes have been also been determined which may suggest a binding mode for cytoplasmic iron sulfur and helicase-type proteins on HEAT repeats of MMS19.
MMS19 could determine selectivity among the overall set of iron sulfur apoproteins if only those interacting with DNA (or comparable nucleotide) have binding propensity for HEAT units. This specificity would then vary by organism, as would the effects of knock-in replacement. Only those apoproteins that align along the linear scaffolding structure in close enough proximity to CIA effector proteins receive an iron sulfur complex. Not every protein arising in this context need directly bind a HEAT domain -- they could bind another protein with that capacity.
This scaffolding scenario requires multiple non-homologous proteins to have HEAT binding sites, which seemingly requires convergent evolution on a significant scale since a shared mobile binding domain can be ruled out. If MMM19 is truly a eukaryotic innovation, then cytoplasmic iron assembly complex initially functioned without the scaffolding until MMS19 and these binding sites evolved.
That seems implausible, so some other common ground must account for HEAT binding. One option, based on the super-helical configuration and major groove of the overall ARM domain, supposes that that MMM19 spoofs a DNA helix or nucleotide base in shape and charge (along the lines of W536 in CRY1B photolyase). This would explain most of the specificity -- each archaeal apoproteins of DNA metabolism needing an iron sulfur cluster already has a DNA binding site, so already an appropriate MMM19 HEAT binding site.
In early endosymbiosis, retained bacterial cluster assembly machinery collides with nuclear-encoded archaeal iron-sulfur protein motifs previously maturated by a different system, a conflict that had to be seamlessly resolved without ever a gap in continued functionality.
CIAO1
(coming shortly)
FAM98B
(coming shortly)
IOP1
(coming shortly)
XPD
(coming shortly)